Know the difference, make a difference
for women with aortic stenosis.§
Give your patients with symptomatic severe aortic stenosis (ssAS) the valve performance‡ advantages of Medtronic Evolut™ TAVR.1
Give your patients with symptomatic severe aortic stenosis (ssAS) the valve performance‡ advantages of Medtronic Evolut™ TAVR.1
Failure to seek timely treatment for ssAS is a problem that can cost patients their lives. If left untreated, once severe symptoms develop, average patient survival is just 50% at 2 years.2
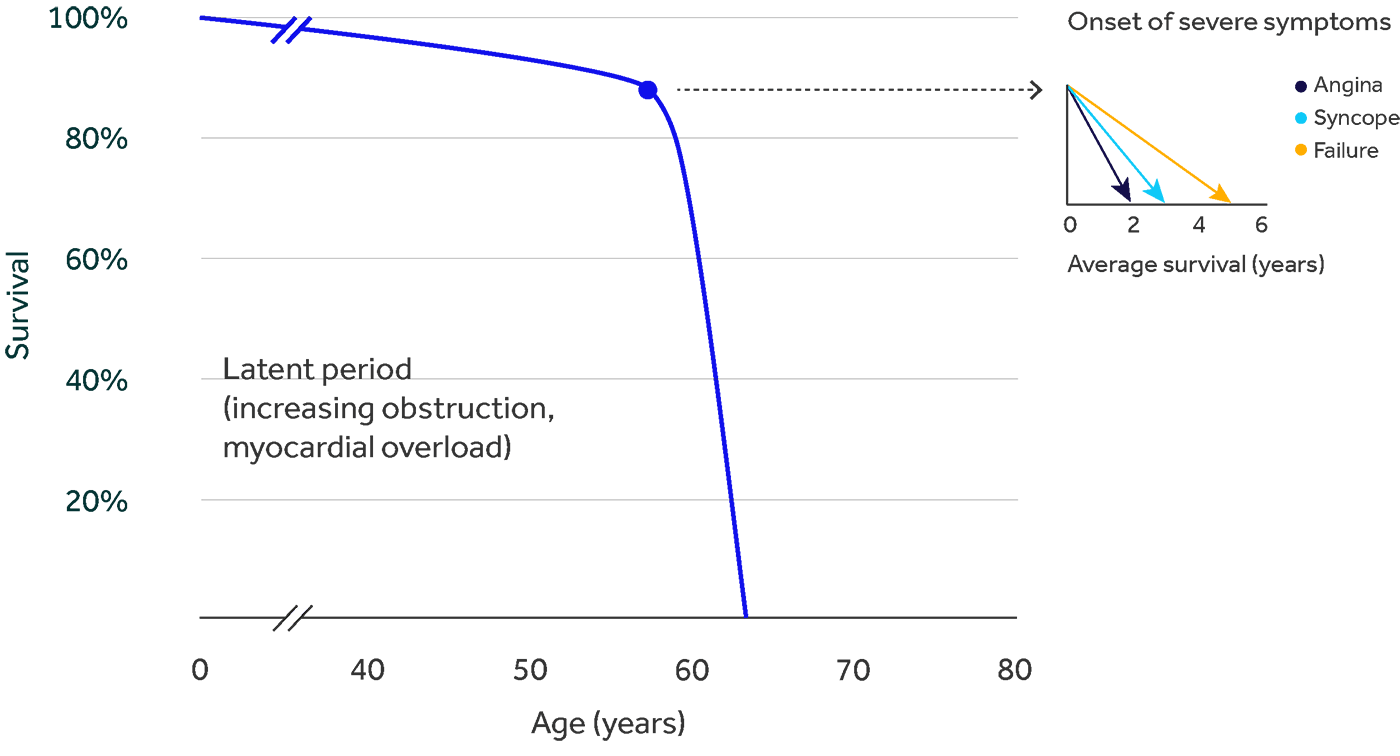
Diagnosing ssAS can be more challenging in women compared to men, because their symptoms appear differently, and the severity is more frequently underestimated.3,4 Review the common diagnostic challenges of ssAS and identify strategies to improve treatment effectiveness.


When it comes to heart valve disease, research shows that aortic stenosis (AS) impacts women differently than men, which must be addressed to ensure timely treatment and save lives.3,4

The SMART Trial is the largest, most rigorous trial to date, to randomize patients to the two most widely used TAVR devices, and the largest TAVR trial to enroll predominantly women.1
The SMART Trial enrolled 87% women1
When TAVR is the best option, it can make a difference in overall health outcomes. Based on the initial SMART results, the data points to Evolut™ TAVR — proven for women.†,1,7,8
Evolut™ TAVR delivers superior valve performance‡ in women with a small annulus¶ at one year versus SAPIEN™* platform.1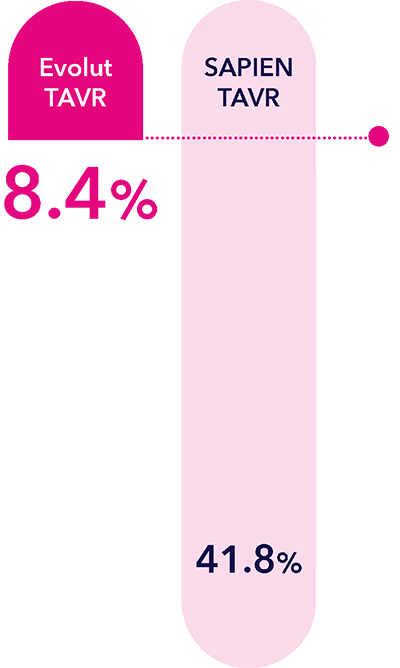
Sustained valve performance, as defined as freedom from bioprosthetic valve dysfunction (BVD◊), helps keep your patients alive and out of the hospital.9
If women do receive TAVR treatment, they have superior valve performance‡ compared to the SAPIEN™* platform at 1 year, based on results from the SMART trial.1

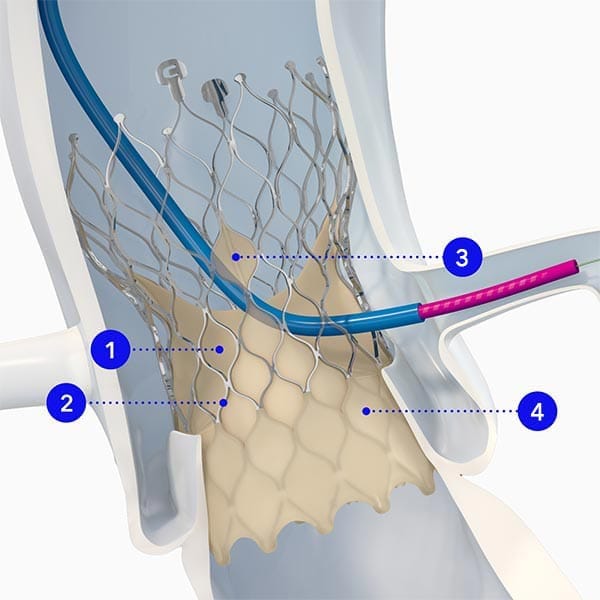
Built on the original CoreValve™ platform, Evolut™ TAVR is engineered with specific design elements to promote sustained valve performance with consistently large EOAs and low gradients over time.10,11
Durability starts with design.
1Supra annular positioning
2Nitinol frame
3Four times larger windows
4External tissue wrap
Give your ssAS patients a proven treatment option. When you believe TAVR is the best choice for your patients, you may not be thinking of a particular valve. Maybe you should be.
Compared to the SAPIEN™* TAVR valve, Evolut™ TAVR demonstrated significantly better VARC-3∆ ordinal outcomes for quality of life at 12 months.1 Give your TAVR patients, especially women, the chance to benefit from a Heart Team experienced with Evolut™ TAVR.
Provide your patients with educational resources that will help them better understand their ssAS and TAVR as a potential treatment option.


† Based on the 1-year follow-up results from the SMART clinical trial which showed differences in valve performance‡ for Evolut compared to SAPIEN* and no differences in safety outcomes. SMART primarily studied small annulus patients, predominantly women.1 Additional clinical trials on women, regardless of their annulus size, have shown comparable mortality rates in women and men treated with TAVR7 and lower mortality rates for women treated with TAVR compared to women treated with surgical valve replacement8 at 1 year after the procedure.
‡ Valve performance as defined as freedom from bioprosthetic valve dysfunction (BVD) through 12 months. BVD is defined as a composite including any of the following: hemodynamic structural valve dysfunction (mean gradient ≥ 20 mmHg), non-structural valve dysfunction (severe prothesis-patient mismatch or ≥ moderate aortic regurgitation), clinical thrombosis, endocarditis, and aortic valve reintervention.
§ Evolut TAVR is indicated to treat patients who have been diagnosed with symptomatic severe aortic stenosis.
◊ Bioprosthetic Valve Dysfunction12 (BVD) defined as: SVD (mean gradient ≥ 10 mm Hg increase from 30-day/discharge to last echo AND ≥ 20 mm Hg at last echo or new onset/ increase of ≥ moderate intraprosthetic aortic regurgitation), NSVD (severe PPM at 30-day/discharge or severe PVR through 5 years), clinical valve thrombosis, and endocarditis.
¶ In patients with small annuli (area ≤ 430 mm2) in all-comers trial, consisting of majority low surgical risk participants (52.1%).
∆ VARC-3 ordinal outcome: (i) death; (ii) worsened: decrease from baseline >5 points; (iii) no change: change between -5 and <5 points; (iv) mildly improved: increase between 5 and <10 points; (v) moderately improved: increase between 10 and <20 points; (vi) substantially improved: increase ≥20 points.